2852 Alton Pkwy, Irvine, CA 92606
2852 Alton Pkwy, Irvine, CA 92606
INTRODUCTION

Lateral Flow Immunoassays are used by point-of-care markets for
immunological testing. Examples of utilization of immunoassays
include cholesterol, cardiac arrest, diabetes testing. Recently, and due
to some constraining patent issues, many manufacturers of lateral flow
assays have invested into R&D to develop multiplexing assays on
complex platforms for very specific applications, and to improve the
design of current lateral flow substrate to increase reproducibility in
the final product.
Dispensing Considerations for Lateral Flow
The lateral flow immunoassay format was developed in the seventies and within 10 years became a standard platform for a variety of Point of Care (POC) tests. The benefits of this format were:
- Ease of use
- Requires relatively small amount of sample
- Adequate level of sensitivity
- Ease of manufacture in large scale
- Stability of the final product at room temperature (shelf life)
- Ease of implementation with a reader technology
- Ease of approval by regulatory instances (format well know by the market)
- Relatively inexpensive to manufacture
For many tests, this format was very attractive. However, recent market
needs have evolved into higher demands, requiring LFIA tests to produce
more than the traditional “yes/no” result. The progression of lateral flow
tests to true quantitative formats is an active area of current research and
development.
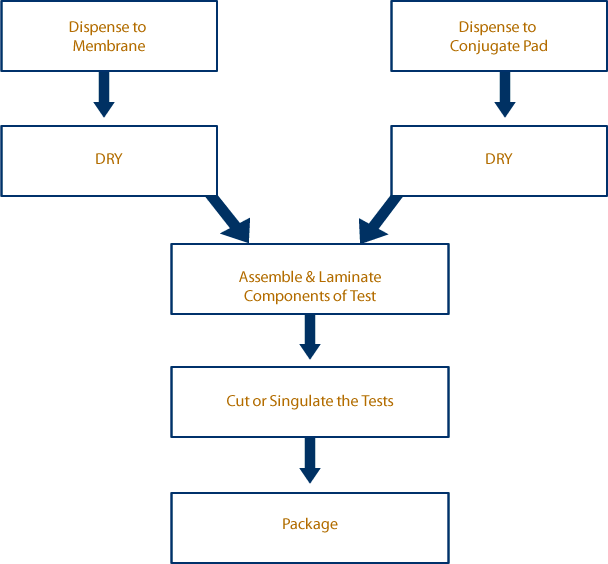
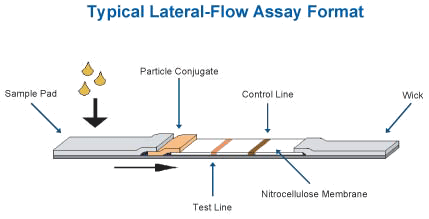
Traditionally, a lateral flow immunoassay device is assembled with a variety
of materials and reagents (Figure 1). Typical components are a backing
card, membrane, sample pad, absorbent pad, conjugate pad, and
possible a wick. The tests are assembled during a “Batch Process” or
an “In Line Process”, the first one being far more common until recent
years.
The batch process starts with a roll of each material; i.e. membrane,
sample pad, conjugate pad, sample pad, wick material, and individual
backing cards. Each material goes through its own process independently
and then once complete the final test is assembled.
The other alternative to the Batch process is the In-Line method. An In
Line process starts with all materials in roll format, and is typically
achieved with three types of modules. The first is the reagent dispensing
and drying module, the second is the slitting module, where
processed webs are cut down to narrower widths for lamination, and
the third module is the actual lamination of the LFIA.
Typically In-Line approaches are designed to meet the specific needs of
an application. The In Line approach allows multi-tasking between both
dispensing and impregnation applications, and has capacity for in-line
drying and quality control monitoring.
Applications Flow Diagram